Learn a little about the history of liver transplantation and exciting emerging areas in the field.
Reference: https://www.universityhealth.com/news/university-health-transplant-institute-named-best-liver-transplant-program-in-country
Listen to Dr. Rodas discuss the management of non-alcoholic fatty liver disease in patients before and after liver transplant (4 mins)
TLI’s own Drs. Lawitz and Poordad were recently recognized for their scientific contributions to the liver field in a published worldwide list of the top 2% of scientists. The impact
SOURCE: https://soundcloud.com/kkvidfw/liver-disease-dr-eric-lawitz?utm_source=clipboard&utm_medium=text&utm_campaign=social_sharing
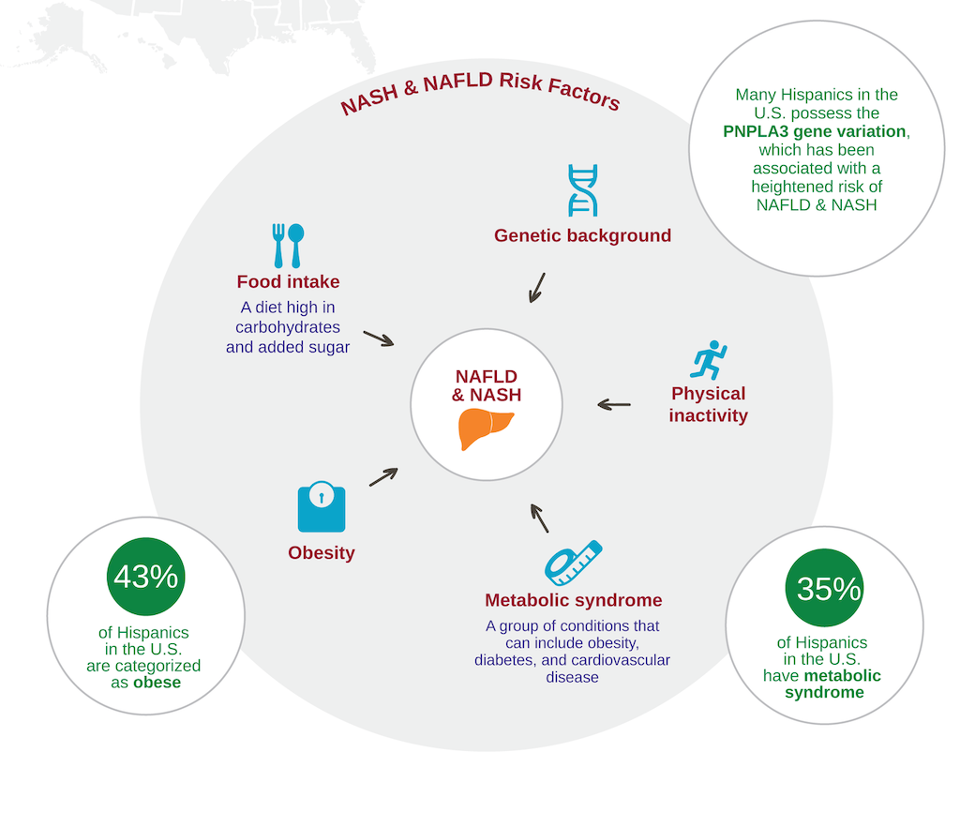
The Latino population is the largest racial and ethnic minority group in the US. With approximately 60.6 million people, it comprises a bit more than 18% of the US population,
“Well, I like to make jewelry,” said Ester LaRita. She is a talented jewelry maker who loves to spend time with her family. But her world changed overnight after one
SOURCE: https://www.tpr.org/podcast/the-source/2021-07-28/hepatitis-c-infections-are-on-the-rise-amid-the-pandemic-half-of-infected-people-dont-know-they-have-it
SOURCE: https://www.tpr.org/podcast/the-source/2021-07-20/americans-are-drinking-more-to-cope-during-the-pandemic-what-are-the-health-risks
In an exclusive video with Healio Gastroenterology, Eric Lawitz, MD, from the Texas Liver Institute, The University of Texas Health, San Antonio, spoke about the NAVIGATE trial in nonalcoholic steatohepatitis-related
La doctora Rita Lepe nos explica cómo detectar a tiempo la hepatitis tipo C y qué tratamiento existe para esta enfermedad que provoca cirrosis del hígado, una condición que cobra
La hepatitis C es una enfermedad causada por un virus que afecta el hígado y puede ocasionar serios problemas de salud, como insuficiencia hepática, cirrosis, cáncer y hasta la muerte.
Written by Pramad Sukumaran | @pramod_ihpr Chronic liver disease can wreak havoc on the body, especially when there is a viral illness spreading worldwide. People suffering from Non-alcoholic Fatty Liver
According to the 2019 US census, Hispanics make up 18.5% of the US population (60 million). In Texas, 39.7% of the population is Hispanic (11.5 million) and they primarily live
Listen to TLI’s Dr. Eric Lawitz discuss the latest advances in fatty liver disease on the Dr. Joe Galati podcast
Congratulations Dr. Fred Poordad for being named 2020 Outstanding Medical Researcher by the San Antonio Business Journal
Dr. Rita Lepe will be seeing patients at the new Dallas clinic 7515 Greenville AveSuite 600Dallas, Texas 75231 Phone : 214-945-2521Fax : 214-945-2524
Bottom Line: With these therapies, only one in 1,000 hepatitis C patient will remain uncured. Sometimes progress seems to happen very slowly…and then all at once. Such is the case with treatments
A disease you may never have heard of is slowly damaging the health of a quarter of Americans. In San Antonio and the Valley, fatty liver disease affects nearly half
By Naim Alkhouri, Fred Poordad, and Eric Lawitz Nonalcoholic fatty liver disease (NAFLD) is considered the hepatic manifestation of insulin resistance, which is the hallmark of type 2 diabetes (T2D). NAFLD
As we conclude another year now past the peak of hepatitis C research, HCV Nextasked me to consider where we came from, where we are now, and where we’re headed.
By Gail Connor Roche Patients with the hepatitis C virus (HCV) who received a 16-week regimen of glecaprevir and pibrentasvir (Mavyret) achieved high sustained virologic response (SVR) rates after previous treatments
BY LIZ HARROUN Due to rising rates of childhood obesity and diabetes in Texas, non-alcohol induced fatty liver disease is the number one need for transplant in young adults. We
Dr. Eric Lawitz, internationally recognized for his work in liver disease, published groundbreaking results from a Phase 3 study in patients with chronic hepatitis C infection and severe renal impairment. The
(Reuters Health) – Nonalcoholic fatty liver disease and its more aggressive form, nonalcoholic steatohepatitis, have become the fastest-growing reasons for liver transplants in young Americans, according to a recent study.
Patients with hepatitis C genotype 3, with or without compensated cirrhosis, achieved significantly high rates of sustained virologic response after treatment with Mavyret and experienced no significant adverse events related
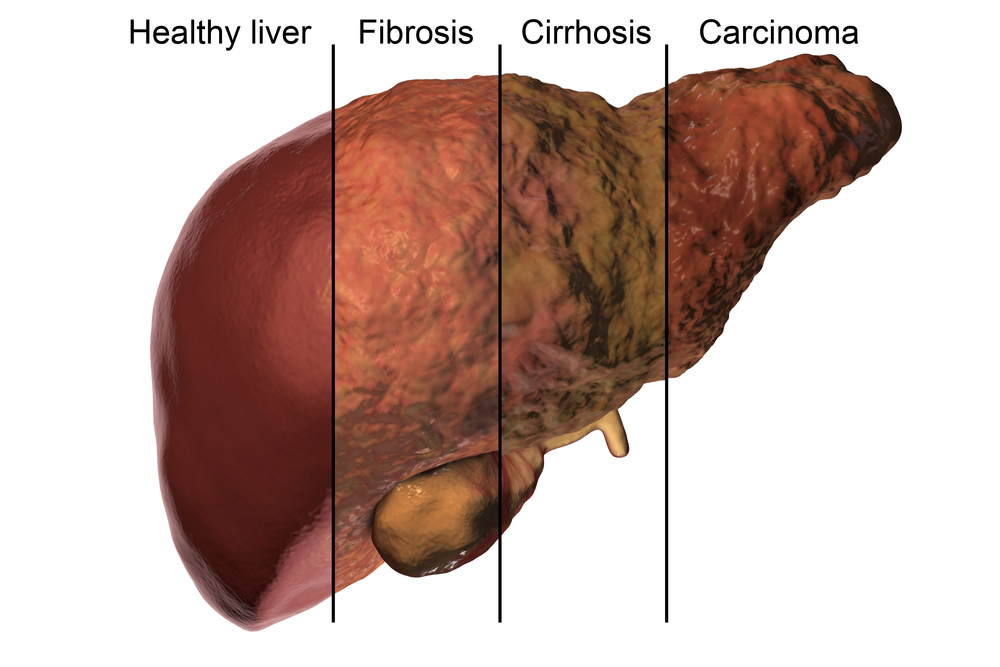
Cirrhosis, or advanced fibrosis of the liver, presents with many challenges such as complications associated with portal hypertension, but in today’s medical climate, it must also be considered in relation
Does liver disease hurt? This is a common question that our hepatologists are asked at the Texas Liver Institute so we decided to answer it! The liver does not contain
Is acetaminophen (Tylenol®) or NSAIDS (ibuprofen or asprin) safe for individuals with liver disease?
Is acetaminophen (Tylenol®) or NSAIDS (ibuprofen or asprin) safe for individuals with liver disease? Contrary to many peoples beliefs, acetaminophen can be used in patients with chronic liver disease (CLD).
PANGENOTYPIC HCV REGIMENS: ALL FOR ONE AND ONE FOR ALL It’s no secret the incredible advancements in Hepatitis C treatment over the past 5 years, but it may come as a surprise
Transient Elastography (FibroScan) Non-Invasive Liver Fibrosis Assessment Accurately staging liver disease is important in the determination for and monitoring of a treatment regimen. A well-studied technology has become available to
Liver disease is serious and can go undetected for years. That is why the Texas Liver Institute offers seminars, free screens and lots more. Watch this video to learn more
Study findings shed light on effective new HCV therapy that will treat patients who have failed other therapies San Antonio, Texas, April 27, 2017 (Newswire.com) – Dr. Fred Poordad, internationally
SAN ANTONIO, /PRNewswire/ — The Texas Liver Institute announced that Dr. Naim Alkhouri has joined the staff as their Director of the Metabolic Center and will have a leadership role in
By Ray Cavanaugh Ray Cavanaugh is a freelance writer from Boston, MA. His interests include history, health topics, and current events in faraway places. They have fatty liver disease, but
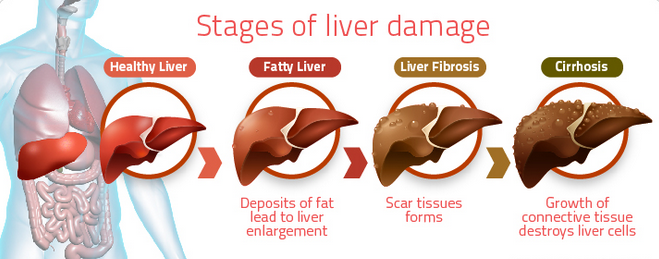
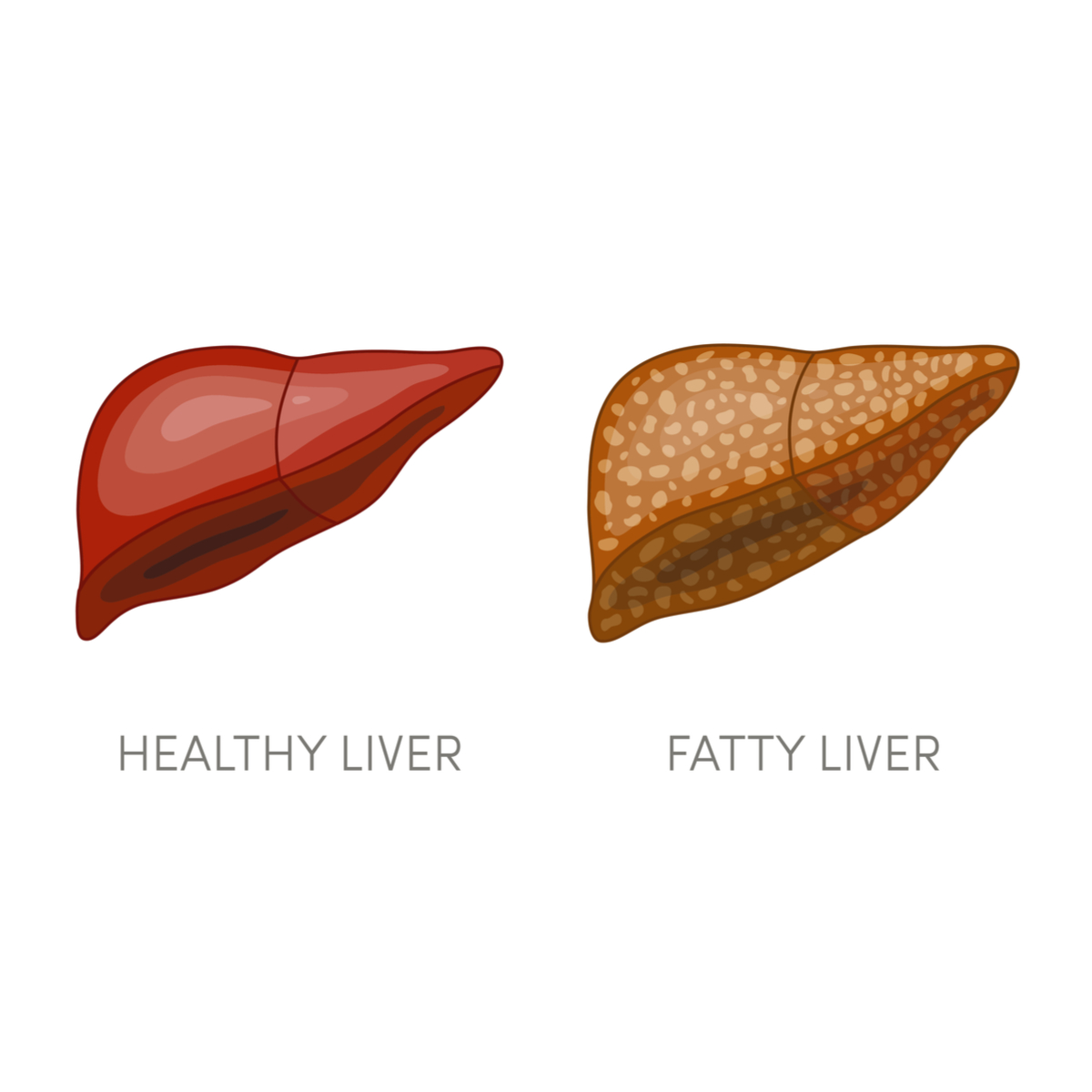
Fatty liver is a condition where the liver becomes infiltrated with fat. Most people with fatty liver are overweight, have diabetes or pre-diabetes.
SAN ANTONIO — Fatty liver disease can be caused by drinking too much, but in many men genetics and diet can trigger the illness. “There are a lot of untapped
Just a few years ago the outlook for treating patients with chronic hepatitis C was grim. For almost a year, patients would receive a complicated regimen of shots and up
Watch the Dr. Phil interview with Dr. Poordad
BARCELONA — Combination therapy with ABT-493, a novel NS3/4A protease inhibitor, and ABT-530, a pangenotypic NS5A inhibitor, produced sustained virologic response rates around 90% in a cohort of treatment-experienced patients,
BARCELONA — An investigational drug combination offers renewed hope of a cure for chronic hepatitis C (HCV) patients who have tried and failed therapy with the new direct-acting antiviral agents,
BARCELONA — A new three-pronged approach to hepatitis C virus effectively treated prior non-responders with genotype 1, according to a presenter at the International Liver Congress. “As increasing numbers of
BARCELONA — In this video perspective from the International Liver Congress, Fred Poordad, MD, discusses the combination treatment of ABT-493 and ABT-530 (AbbVie) for patients who previously failed direct-acting antiviral
– 95 percent of patients achieved SVR12 with 12 weeks of ABT-493 and ABT-530 with and without RBV in GT1 chronic HCV infected patients without cirrhosis who failed previous therapy
AbbVie (NYSE: ABBV) announced that 91 percent (n=20/22) of genotype 1 (GT1) chronic hepatitis C virus (HCV) infected patients who failed previous therapy with direct-acting antivirals (DAAs) achieved SVR12 with
http://medpagetoday.s3.amazonaws.com/media/sponsors/hothcv/Pipeline2.mp4 MedPage Today invited specialists from leading medical institutions to weigh in on the latest advancements in hepatitis C with one question each day for 10 days. In this installment,
The U.S. Food and Drug Administration has granted amended Breakthrough Therapy Designation for an investigational combination of drugs that show great promise for treating the sickest hepatitis C patients—those with
MedPage Today invited specialists from leading medical institutions to weigh in on the latest advancements in hepatitis C with one question each day for 10 days. In this installment, we
At the time, the medication Joey was taking kept the disease from progressing. But it had debilitating side effects. “I would feel the side effects like days after. It would
A medical breakthrough tested in San Antonio is opening doors for patients diagnosed with hepatitis C. The virus can be deadly, but now researchers say they’ve found a cure for
Treatment-naive and experienced patients with chronic hepatitis C virus genotype 1 infection without cirrhosis achieved a sustained virologic response at 12 weeks while on a fixed-dose regimen of daclatasvir, asunaprevir
97% of post-transplant patients with HCV genotype 1a achieved cure 91% of post-transplant patients with HCV genotype 3 achieved cure No need seen to alter existing transplantation medication regimens
Merck has released first presentations of data from C-EDGE phase III clinical trial of grazoprevir/elbasvir in patients with or without cirrhosis who are infected with chronic hepatitis C virus (HCV)
Janssen Sciences Ireland UC, one of the Janssen Pharmaceutical Companies of Johnson & Johnson (Janssen), announced results for its hepatitis C treatment simeprevir at The International Liver Congress™ 2015. New
Bidness Etc takes a look at how Merck’s new drug is different from the currently available drugs by Gilead and AbbVie and what analysts think about the competition it can
More than 70% of people with chronic genotype 1 hepatitis C who did not achieve a sustained virological response when treated with sofosbuvir/ledipasvir (Harvoni) for 8 or 12 weeks were
An interferon-free regimen of sofosbuvir, daclatasvir and ribavirin for 12 weeks produced sustained response rates of 83% for people with hepatitis C virus (HCV) who had advanced liver cirrhosis and
VIENNA — An investigational drug combination had high efficacy among patients with cirrhosis associated with hepatitis C (HCV) or recurrence of the disease after a transplant, researchers said here. But
With Merck & Co. poised to disrupt competitors with new hepatitis C drugs, rivals are scrambling to get ahead the best way they can figure: make a medicine that works
Results of trials of antivirals to treat hepatitis C infections are dominating the proceedings at the 2015 International Liver Congress in Vienna, Austria. In a presentation today, Fred Poordad, MD,
The use of interferon to treat hepatitis C infections has fallen out of favor with the advent of new antivirals and drug combinations that have fewer side effects. But in
VIENNA — Patients with cirrhosis as well as genotype 1 and genotype 3 HCV responded favorably to 8 weeks of treatment with a novel grazoprevir and elbasvir-based combination regimen, according
VIENNA — Twenty-four weeks of treatment with Harvoni yielded a 12-week sustained virologic response rate higher than 70% in a cohort of patients who had failed previous therapies, according to
UT Health Science Center San Antonio doctor presents results of daclatasvir regimen SAN ANTONIO (April 30 2015) — A number of new, highly effective oral treatments for various types of
Merck & Co. said Friday that its experimental drug combination for treating chronic hepatitis C patients demonstrated a 95% effective rate in a late-stage study. The study combined grazoprevir and
A number of new, highly effective oral treatments for various types of hepatitis C have been approved in the past few years. However, two groups who have not benefitted from
Boston—Can treatment of hepatitis C infection with new direct-acting antivirals (DAAs) be shortened to less than 12 weeks? Interim results from a recent trial suggest treatment durations of eight or
Following this program, participants should be better able to: Use in a clinical setting important findings from key studies on CHC diagnosis and treatment presented at AASLD 2014 Employ current treatment protocols
In a phase 2a clinical trial, treatment with beclabuvir and a combination of pegylated interferon alfa-2a and ribavirin was well tolerated among patients with hepatitis C virus genotype 1 infection,
Multidrug Regimen Seen Challenging Recently Approved Drugs from Gilead Sciences U.S. regulators on Friday approved AbbVie Inc.’s new multidrug regimen for hepatitis C, the latest in a wave of new
– In Phase 3 clinical trials, VIEKIRA PAK cured 95-100 percent of hepatitis C patients, with less than 2 percent of patients experiencing virological failure – Tolerability profile shows more
It’s becoming health threat at young age When David Martinez was diagnosed with advanced cirrhosis of the liver, he was surprised and confused. “I thought cirrhosis came from drinking,” said
Two studies suggest drugs could yield high cure rates. A new study suggests that some subgroups of patients with hepatitis C (HCV) infection can achieve sustained virologic responses (SVR) in
Can outcomes be sustained with shorter regimens? A new study suggests that some subgroups of patients with hepatitis C virus infectioncan achieve sustained virologic responses (SVR) in as short as
S. Texas Latinos have nation’s highest rate Experts are sounding the alarm on liver cancer, which has reached epidemic levels in South Texas, likely because of the region’s high rates
We got an overview on this important session at The Liver Meeting® 2014 which aims to investigate the latest on clinical issues commonly seen in practice. Diagnostic strategies and approaches
A San Antonio doctor has published a cure for hepatitis C, one of the most painful, and expensive, diseases in the world, News Radio 1200 WOAI reports. Dr. Eric Lawitz,
San Antonio researcher leads national study that included hard-to-treat patients SAN ANTONIO ― Researchers from The University of Texas Health Science Center at San Antonio, the Texas Liver Institute and
Miami, Florida — New data offers hope to liver transplant patients infected with the hepatitis C virus. This virus may lead to liver destruction and cancer in up to 40% of the
A new study published in the New England Journal of Medicine and presented at the International Liver Congress highlights the use of a multitargeted therapy that combines the drug ABT-450 with
A San Antonio researcher has announced that he has found a cure for Hepatitis C. In the first-of-its-kind study dedicated to patients with Hepatitis C and cirrhosis of the liver,
Multitargeted therapy of ABT-450 with ritonavir, ombitasvir and dasabuvir yielded strong response rates with few discontinuations among patients with cirrhosis and hepatitis C genotype 1 treated for 12 or 24
A drug combination with striking efficacy in relatively healthy hepatitis C (HCV) patients had similar outcomes in patients with compensated cirrhosis, a researcher said. In an open-label phase III trial,
UT Medicine San Antonio & Texas Liver Institute Researchers Cure 90% of Studied Hepatitis C Patients
An international study including researchers from University of Texas Medicine San Antonio and Texas Liver Institute announced significant success in the treatment of hepatitis C infection in patients with liver cirrhosis.
Oral combination proves safe for patients who could not have interferon therapy SAN ANTONIO, Texas — A novel 12 week regimen of pills has cured more than 90% of hepatitis
An experimental trial of two drugs in one pill had a high cure rate for patients infected with hepatitis C. A new drug combination has effectively cured hepatitis C in